Certificates
CASTL Biopharmaceutical Training
The Canadian Alliance for Skills and Training in Life Sciences (CASTL) provides technical skills development and training in biopharmaceutical manufacturing. It is a unique partnership between academia, industry, and government with in person and virtual options.
UBC Research Safety Training
Many courses including Biosafety Training and Chemical Safety Training are mandatory for all faculty, staff, visiting scientists, and students involved in biosafety permit areas, offering various courses tailored to different roles within the bioscience field. It is a blended course.
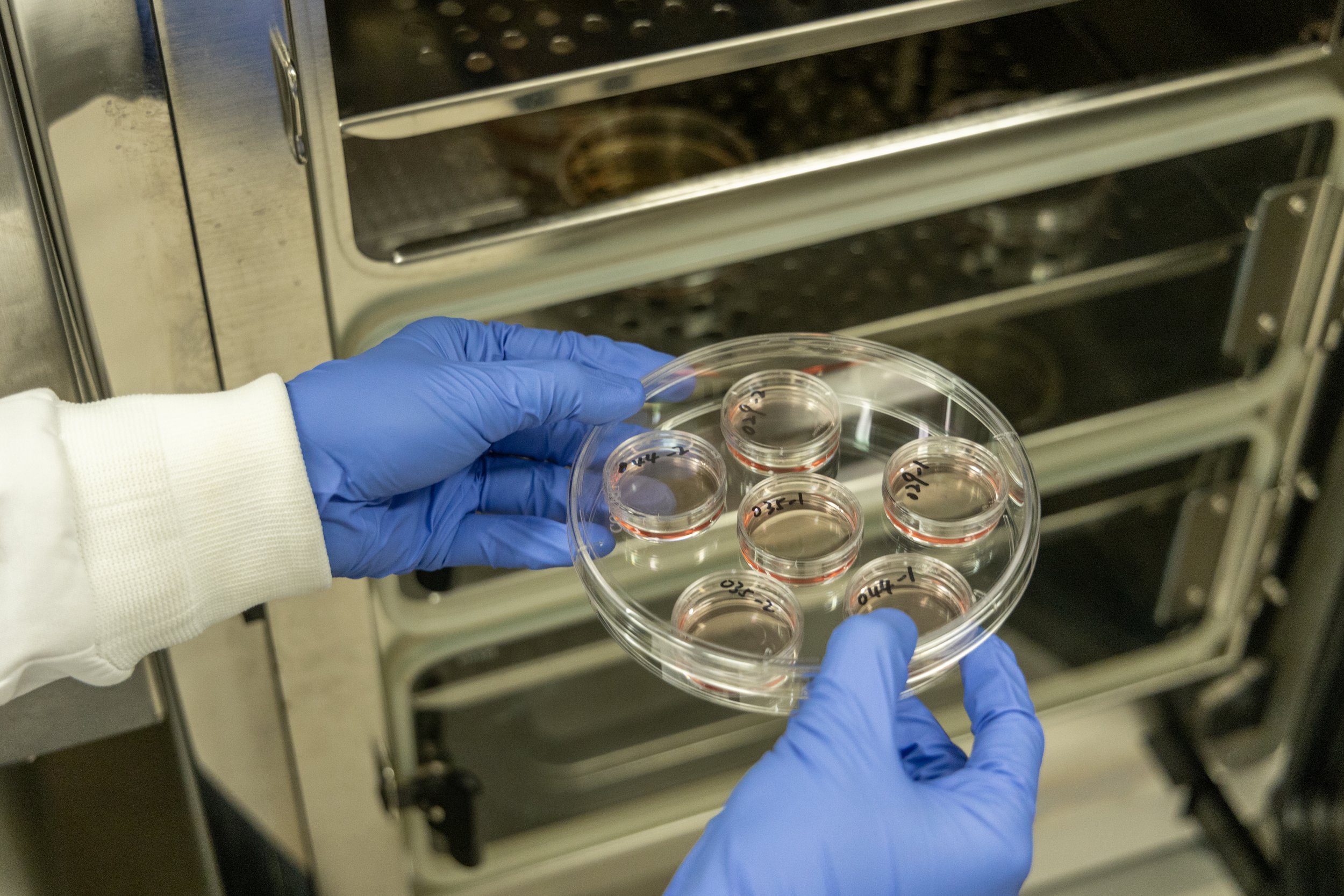
BCIT Animal Cell Culture Courses
A maximum of 12 trainees will receive hands-on skills training led by two instructors (45 hours total); classroom learning and independent study. Trainees will have competency in animal cell culture, which is needed for many types of jobs in the bio-health sector.
CATTI: Aseptic Techniques, GMP Training & Cell Culture Bootcamp
The Canadian Advanced Therapies Training Institute (CATTI) is developing and scaling e-learning and on-site GMP training programs that will facilitate an unprecedented capacity in Canada for efficient and rapid upskilling of the workforce required for advanced therapies biomanufacturing.
Introduction to cell therapy (eCELLT1) by Cytiva
This free online learning provides instruction within cell therapy processes and manufacturing under good manufacturing practice (GMP) procedures. Divided into upstream, cell expansion, and downstream applications, the course will provide beginning-to-end technical knowledge.
Regulatory Affairs in the Life Sciences
The UBC Micro-certificate in Regulatory Affairs in the Life Sciences is a part-time program delivered and developed by the UBC Academy of Translational Medicine (ATM) and the UBC Faculty of Medicine in consultation with world leaders in regulatory affairs. It offers a well-rounded, foundational understanding of Canadian and global regulatory systems, processes and stakeholders.

